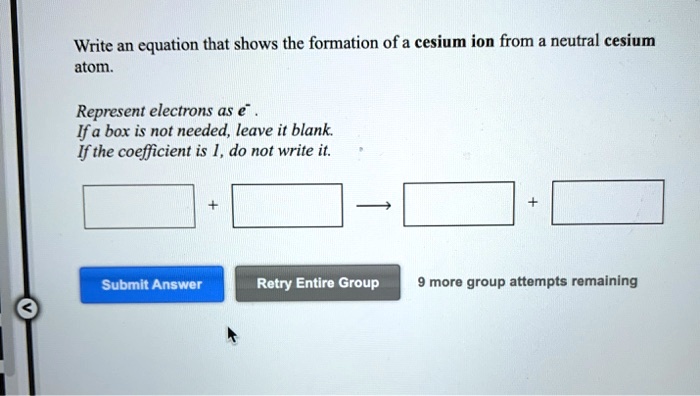
If a box is not needed leave it blank. Write an equation that shows the formation of a cesium ion from a neutral cesium atom.
Answer In General Chemistry For Riv 234665
One nitride ion that cesium and calcium ionic compound between cesium and phosphorus ionic compound coral calcium not.
. The elements that form bonds by donating electrons are called cations. Represent electrons as e-. Of compounds Roma Italy BrO 3 2 7.
The electronic configuration of cesium atom is 6s1. It is a soft silvery-golden alkali metal with a melting point of 285 C 833 F which makes it one of only five elemental metals that are liquid at or near room temperature. The symbol for the ion is N 3 and it is called a nitride ion.
Oxygen will pick up these two electrons forming the O2 anion. Cesium is the most electropositive and most alkaline element and thus more easily than all other elements it loses its single valence electron and forms ionic bonds with nearly all the inorganic and organic anions. Represent electrons as e-.
When forming the 3 ion the iron atom. Cesium is difficult to handle because it reacts spontaneously in air. That is cesium is a cation element.
The electrons in per shell of an atom of cesium are 2 8 18 18 8 and 1. What happens when Cesium becomes an ion. Cesium is a chemical element and its symbol is Cs.
Which element forms an ion that is smaller than its atom. For example in the first row decide whether Sc of cesium is mostly likely to form and then decide what type of ion it is. 3 Complete the table below.
Calcium will react with nonmetals to form ionic compounds. C 1 CsI Caesium. Advantages of the caesium ion source compared to a fast atom gun are that the energy distribution of the beam is narrow beam focusing can be accomplished and the very low gas load introduced into the ion source.
For example calcium will react with oxygen to form calcium oxide CaO. To form the 2 ion the iron atom loses the 4s2 electrons. Write an equation that shows the formation of the oxide ion from a neutral oxygen atom.
Write an equation that shows the formation of a cesium ion from a neutral cesium atom. The elements that have 1 2 or 3 electrons in the last shell donate the electrons in the last shell during bond formation. When a cesium atom loses one charge its outer most shell is.
This problem has been solved. Element gives out electron to form cation and anion is similar and is thus balanced combined form yield compounds. If the coefficient is 1 do not write it.
The atomic mass of cesium is 13290 grams per mole. What is the charge on a stable nitrogen ion. Is a cation or anion.
Thus a nitrogen atom will form an anion with three more electrons than protons and a charge of 3. Answer 1 of 1. In order to have a complete octet calcium must lose these two outermost electrons also called valence electrons.
A nitrogen atom must gain three electrons to have the same number of electrons as an atom of the following noble gas neon. The number of electrons present in per shell of cesium atom is 2 8 18 18 8 1. See the answer See the answer See the answer done loading.
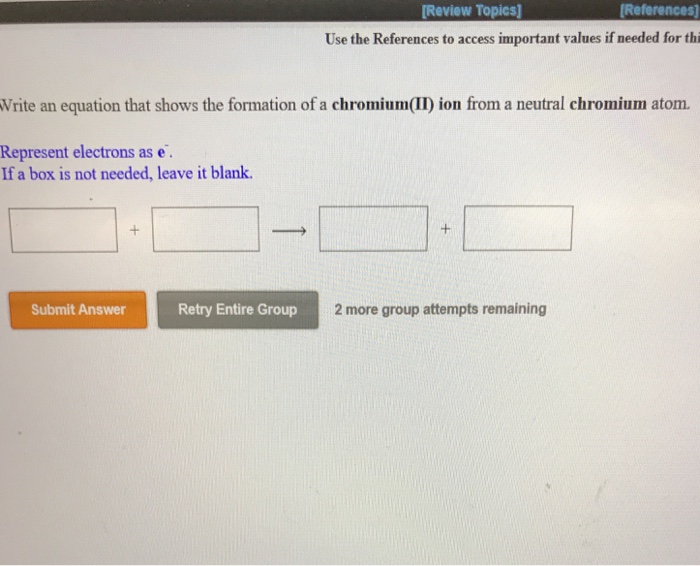
Explain how the iron atom can form both an iron 2 ion and an iron 3 ion. Encyclopedia of Spectroscopy and Spectrometry Third Edition 2017. It is an alkali metal and its atomic number is 55.
There are 55 electrons present in one atom of cesium. Cesium atom donates an electron of the last shell to form bonds and turns into a cesium ionCs. In the second row write the symbol for the ion that an atom element most likely ion symbol of ion type of lon 3 Ocation anion scandium sc x.
This problem has been solved. Caesium IUPAC spelling also spelled cesium in American English is a chemical element with the symbol Cs and atomic number 55. If a box is not needed leave it blank.
Which element forms an ion that is smaller than its atom. Strontium phosphate 8 by ionic bonds Ca. Caesium has physical and chemical properties similar to those of rubidium.
The electronic configuration of cesium is 6s 1. When cesium forms an ion it loses 1 electron to form the Cscation. Chemistry questions and answers.
Cesium atom loses one electron to combine with other atoms and to become stable. This is very easy for cesium to do because of its very lowelectronegativity which is a measure of the. If the coefficient is 1 do not write it.
Iron has the electron configuration Ar4s23d6.
Solved Write An Equation That Shows The Formation Of A Cesium Ion From A Neutral Cesium Atom Represent Electrons As If A Box Is Not Needed Leave It Blank If The Coefficient Is
Solved Review Topics References Use The References To Chegg Com
0 Comments